Click here to view Full Prescribing Information
Kit for the Preparation of Tc99m Exametazime for leukocyte labeling
Indication
Drax Exametazime is a radioactive diagnostic agent indicated for leukocyte (white blood cell) labeled scintigraphy as an adjunct in the localization of intraabdominal infection and inflammatory bowel disease.
Product overview
Leukocyte labeling scintigraphy helps physicians localize intra-abdominal infection and inflammatory bowel disease. Pelvic and abdominal imaging with a gamma camera will show an accumulation of radioactivity in the bowel in early images [less than 4 hours] with increasing intensity and/or no evidence of changing location secondary to GI motility, which is an indication of inflammatory bowel disease or infection. Radioactivity from hepatic excretion detected in the bowel 4 hours post-injection and changing in GI location on serial/subsequent images is indicative of normal GI transit.
Description
Drax Exametazime is a kit containing five (5) single-dose vials. Each 10 mL vial is used to prepare a radioactive diagnostic agent and contains a sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime, 7.6 mcg stannous chloride dihydrate and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure.
When reconstituted with the technetium Tc 99m eluate, each vial will contain a clear, colorless, and foreign particles-free solution of 370 MBq up to 2000 MBq (10 mCi up to 54 mCi). The radioactive solution produced will be used for leukocyte labeling before intravenous administration to the patient.
When Tc 99m pertechnetate in Sodium Chloride Injection, USP (0.9%) is added to Drax Exametazime vial, a Tc 99m complex of exametazime is formed.
Contraindications
None
Warnings and Precautions
Hypersensitivity Reactions: Hypersensitivity reactions, including serious signs and symptoms of anaphylaxis, following administration of Tc 99m labeled leukocytes prepared using Tc 99m exametazime have been reported. Always have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions.
DISCLAIMER:
This information is not intended as medical advice. Responsibility for patient care resides with the healthcare professional on the basis of his or her professional license, experience and knowledge of the patient. For full Prescribing Information including indications, contraindications, warnings, precautions and adverse events, please see the appropriate product labeling.
Full Prescribing Information
Click here to view Full Prescribing Information
Use in Special Populations
Pregnancy
Limited available data with technetium Tc 99m exametazime use in pregnant women are insufficient to inform a drug associated risk for major birth defects and miscarriage. Technetium Tc 99m exametazime is transferred across the placenta. Animal reproduction studies with technetium Tc 99m exametazime have not been conducted. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Pediatric Use
Safety and efficacy in pediatric patients have not been assessed.
Renal impairment
Increased radiation exposure in patients with impaired renal function. A reduction in administered Tc 99m can be considered provided adequate numbers of labeled WBCs are administered
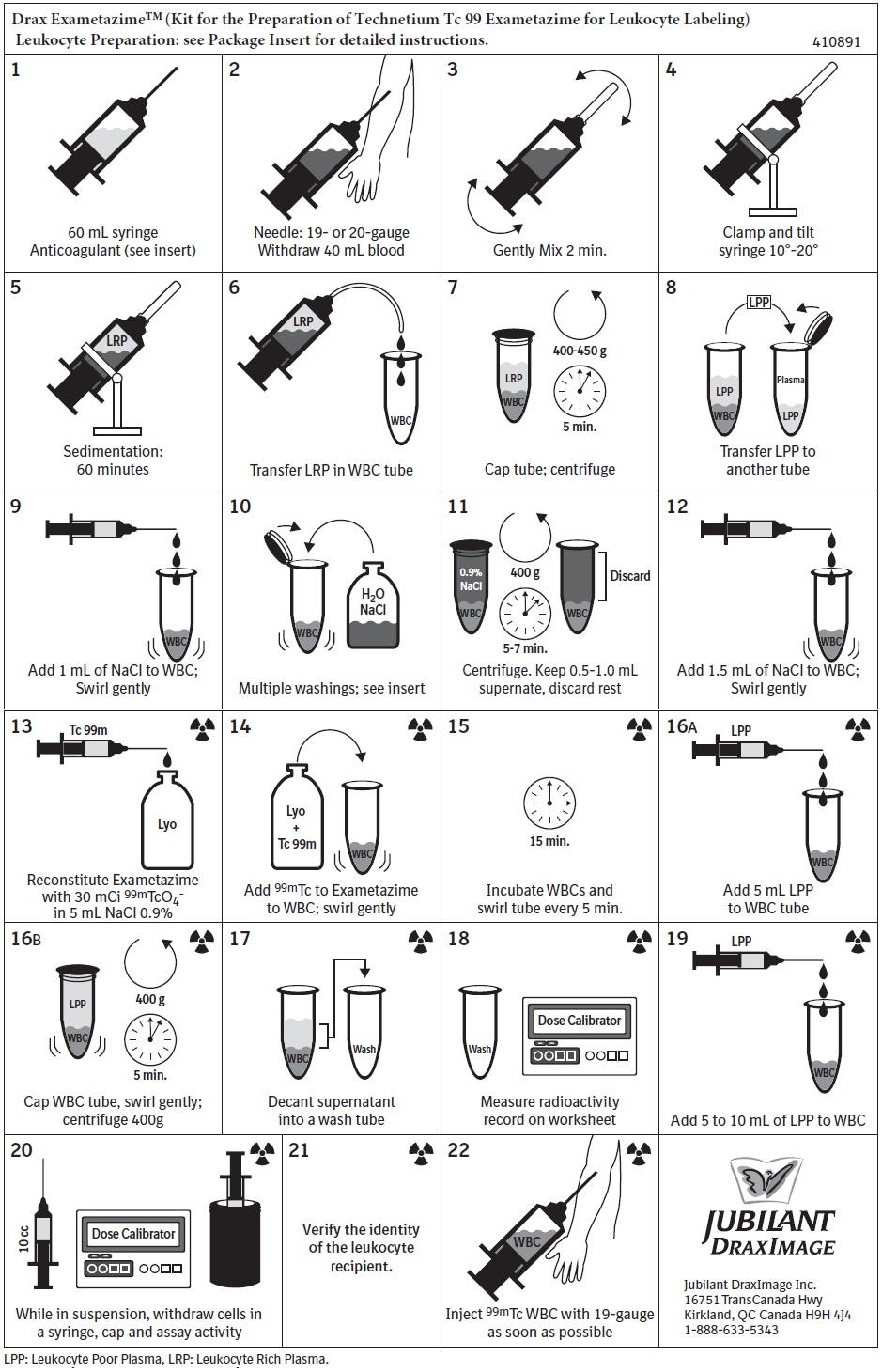
Instructions for Use
Please see Full Prescribing Information for complete Instructions
Safety Data Sheet
Click to view SDS