Indication
Click here to view the Package Insert
Kit for the Preparation of Tc99m Exametazime for leukocyte labeling

Tc 99m Exametazime is an effective agent for in vitro Tc 99m leukocyte radiolabelling. Tc 99m labelled leukocytes are useful in the detection of sites of focal infection, especially abdominal abscess and as an adjunct in the investigation of pyrexia of unknown origin (PUO), and in the evaluation of inflammatory conditions not associated with infection such as inflammatory bowel disease (IBD).
Product overview
Leukocyte labeling scintigraphy helps physicians localize intra-abdominal infection and inflammatory bowel disease. Pelvic and abdominal imaging with a gamma camera will show an accumulation of radioactivity in the bowel in early images [less than 4 hours] with increasing intensity and/or no evidence of changing location secondary to GI motility, which is an indication of inflammatory bowel disease or infection. Radioactivity from hepatic excretion detected in the bowel 4 hours post-injection and changing in GI location on serial/subsequent images is indicative of normal GI transit.
Description
Drax Exametazime is a kit containing five (5) single-dose vials. Each 10 mL vial is used to prepare a radioactive diagnostic agent and contains a sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime, 7.6 mcg stannous chloride dihydrate and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure.
When reconstituted with the technetium Tc 99m eluate, each vial will contain a clear, colorless, and foreign particles-free solution of 370 MBq up to 2000 MBq (10 mCi up to 54 mCi). The radioactive solution produced will be used for leukocyte labeling before intravenous administration to the patient.
When Tc 99m pertechnetate in Sodium Chloride Injection, USP (0.9%) is added to Drax Exametazime vial, a Tc 99m complex of exametazime is formed.
Contraindications
None
Warnings and Precautions
The possibility of hypersensitivity including serious signs and symptoms of anaphylaxis should always be considered. Advanced life support facilities should be readily available. Care should be taken when handling blood specimens to be labelled using this radiopharmaceutical. All human blood samples should be considered potentially infectious. Precautions for handling are as those for handling radioactive materials.
Use in Special Populations
Pediatric Use
Adequate studies do not exist to support the use in children. As in pregnancy and lactating mothers, the benefits to risk ratio should be assessed before consideration is given to the use of this product in this age group.
Use in Pregnancy
In women of childbearing age the possibility of pregnancy should always be taken into account. It would be prudent to treat as pregnant any woman of reproductive age presenting for a nuclear medicine examination at a time when a menstrual period is overdue or missed, unless there is information that precludes pregnancy. If the menstrual cycle is irregular, a pregnancy test may be indicated before proceeding.
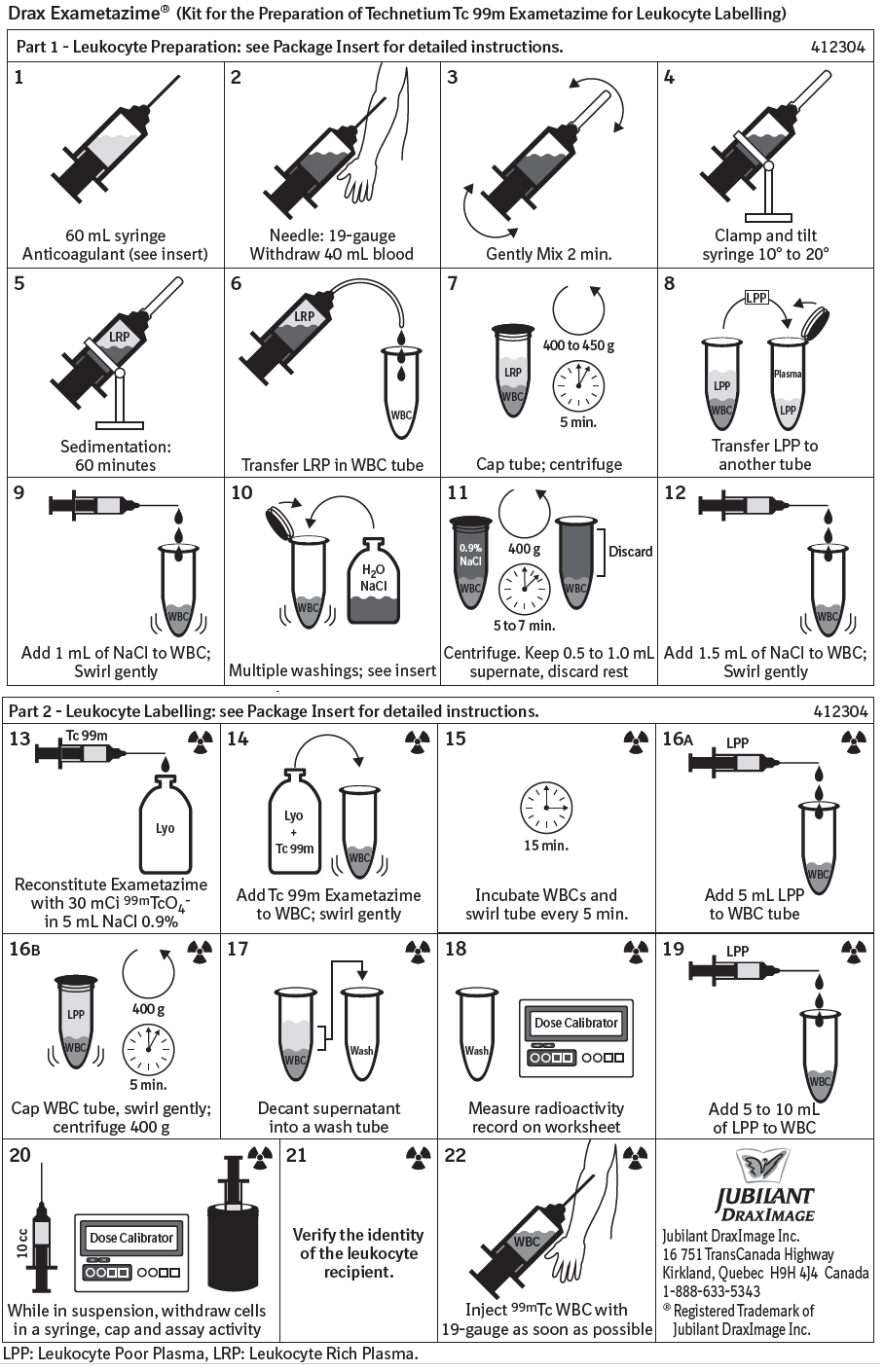
Instructions for Use
Please see Leukocyte Labelling Schematic for complete instructions
Product Insert
Click here to view the Product Insert
Safety Data Sheet
Click here to view SDS