Prescribing Information
Click here to view Full Prescribing Information Including Boxed Warning
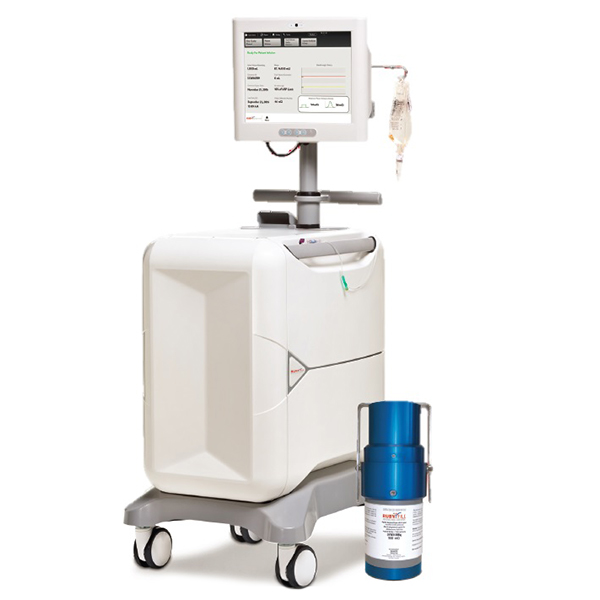
The RUBY-FILL® Rubidium Rb 82 Generator contains accelerator produced Strontium-82, which decays to Rubidium-82. When the generator is eluted with saline it produces a sterile, non-pyrogenic solution of Rb-82 Chloride used for Cardiac Positron Emission Tomography (PET), a non-invasive imaging procedure of the myocardium, to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease.
Due to the short half-life (75 s) of Rb-82, the use of an elution system is required for delivery of the Rb-82 Chloride into a patient for the purposes of performing Myocardial Perfusion Imaging with PET. The Rubidium Elution System has been exclusively designed for use with the RUBY-FILL® Rubidium 82 Generator and to deliver accurate doses of Rb-82 Chloride to patients.
Indication for Use
RUBY-FILL is a closed system used to produce rubidium Rb 82 chloride injection for intravenous use. Rubidium Rb 82 chloride injection is a radioactive diagnostic agent indicated for Positron Emission Tomography (PET) imaging of the myocardium under rest or pharmacologic stress conditions to evaluate regional myocardial perfusion in adult patients with suspected or existing coronary artery disease
Safety and Efficiency
Safety
-
Automated safety alerts that help minimize risk of strontium breakthrough
-
System lockout denies access to patient infusion controls if quality control is not completed or if strontium levels are elevated
-
No need to record dose calibrator readings or manually calculate strontium breakthrough detection
Efficiency
-
Automated quality control and reports
-
Enhanced maneuverability
-
Stick-on printed patient infusion labels
-
30 L generator volume
Precision Driven
Engineered for User Confidence & Efficiency
-
30L generator volume for flexible capacity
-
Long 60-day shelf life
-
Enhanced maneuverability
Accurate Patient-Specific Infusion Options
-
Weight-Based Dosing
-
Constant-Activity Options
-
Reduces test-retest Variability*
Reference:
*Klein R, Ocneanu A, Renaud JM, et al. Consistent tracer administration profile improves test-retest repeatability of myocardial blood flow quantification with 82RB dynamic PET imaging. J Nucl Cardiol. Epub 2016.
DISCLAIMER:
This information is not intended as medical advice. Responsibility for patient care resides with the healthcare professional on the basis of his or her professional license, experience and knowledge of the patient. For full Prescribing Information including indications, contraindications, warnings, precautions and adverse events, please see the appropriate product labeling.
Warning: High Level Radiation Exposure with use of incorrect eluent and failure to follow quality control testing Procedure
Please see full prescribing information for complete boxed warning
High Level Radiation Exposure with Use of Incorrect Eluent
Using the incorrect eluent can cause high Strontium (Sr 82) and (Sr 85) breakthrough levels (5.1)
-
Use only additive-free 0.9% Sodium Chloride Injection USP to elute the generator (2.5)
-
Immediately stop the patient infusion and discontinue the use of the affected RUBY-Full® generator if the incorrect solution is used to elute the generator (4)
-
Evaluate the patient's radiation absorded dose and monitor for the effects of radiation to critical organs such as bone marrow (2.9)
Excess Radiation Exposure with Failure to Follow the Quality Control Testing Procedure
Excess radiation exposure occurs when the levels of Sr 82 or Sr 85 in the Rubidium Rb 82 Chloride injection exceed specific limits (5.2)
-
Strictly adhere to the generator quality control testing procedure (2.6)
-
Stop using the generator if it reaches any of its Expiration Limit (2.7)
RUBY-FILL: Reach Even Greater Heights
Safety Data Sheet
Click here to view SDS.
Request Information
It’s Easy to Get Started with RUBY-FILL
To learn more about the latest advancements in Cardiac PET imaging contact customer service at customerservice@jdi.jubl.com or enter your contact information here: